What’s so interesting about how disposable gloves are made? Quite a lot, it seems.
We frequently get questions about what’s involved in making gloves. We’ve also published a few blog posts over the years describing the manufacturing process, and they’re always among our most popular.
Thinking it might be handy to tie it all together in one, here goes with an easy-to-read summation of how the process works.
The basic building blocks
There are four primary materials used in making disposable gloves: nitrile butadiene rubber, natural rubber latex, polyvinyl chloride, and polyethylene. Various specialty gloves incorporate other materials, but those four cover everything that AMMEX sells.
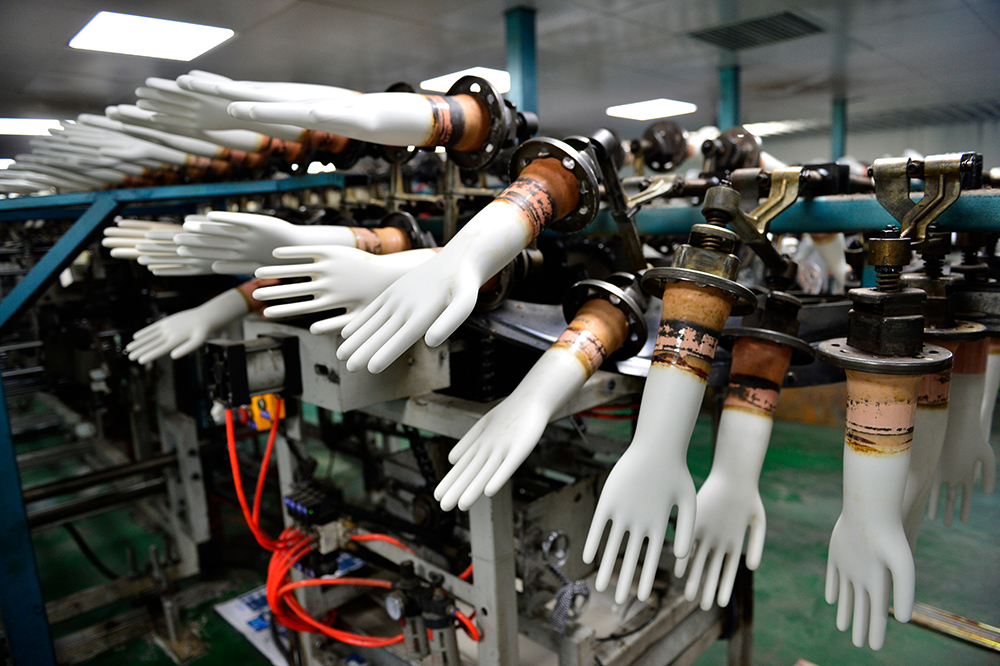
- In glove production, ceramic or aluminum hand-shaped molds, or formers, are first washed in hot water and chlorine to remove all residue.
- Next the formers, suspended on a continuous moving chain, are dipped into a mixture of calcium nitrate solution and calcium carbonate—the nitrate is a coagulant, while the carbonate helps the gloves release from the formers.
- After drying, the molds are dipped into the latex compound, with the duration of the dip determining the mil thickness of the gloves.
- The freshly molded gloves are next leached in a mixture of hot water and chlorine, which removes residual latex proteins and chemicals to help reduce the severity of any allergic reactions to latex.
- The gloves are then dried and cured, then vulcanized to convert the gloves to an elastic state by causing a reaction between rubber molecules in the latex and chemicals that have been added. This gives gloves their elasticity so they are less likely to tear.
- After drying, the gloves are rinsed again to leach out more latex proteins, then the cuffs are beaded, or rolled, to make them easier to don and doff.
Latex is the oldest and most familiar material. In the glove world, it goes back to 1889 at Johns Hopkins Hospital in Baltimore. William Stewart Halstead, the hospital’s first surgeon in chief, is credited with developing the first surgical glove. He petitioned the Goodyear Rubber Company to make gloves because his surgical team’s hands were irritated by the chemicals used.
Latex was the standard for the better part of a century, with disposable gloves becoming the norm in the 1960s. Increased use of latex gloves, particularly during the AIDS crisis of the 1980s, raised awareness of latex allergies and brought about the introduction of vinyl and nitrile gloves by the mid-1990s. Those two materials, which contain no plant proteins to cause allergic reactions, have largely supplanted latex, but it still has a devoted following in some uses.
Where latex comes from
Latex concentrate, the raw material used in manufacturing, comes from Hevea brasiliensis, also known as the Pará rubber tree. It originated in the Amazon rainforest and was introduced to Southeast Asia, Africa, and elsewhere in the 1870s. Today most of the world’s natural rubber comes from plantations in India, Indonesia, Thailand, Malaysia, and Vietnam.
Rubber trees are usually ready to be tapped after about seven years of growth. Thin strips of bark are removed from the tree, which makes the milky-white sap run downward into a cup. After about six hours, the fluid stops, usually filling a gallon bucket.
Because of its high water and non-rubber content—about 70% is water, protein, sterol glycosides, resins, ash, and sugars—the latex is concentrated and stabilized. The latex is mixed with processing chemicals including sulfur, zinc oxide, accelerators, pigments, stabilizers, a de-webbing agent, and antioxidants. It matures for 24 to 36 hours to become ready for dipping.
Where nitrile, vinyl come from
The processes for creating nitrile and vinyl materials is similar. The nitrile butadiene rubber (NBR) used for nitrile gloves is a copolymer, which is a substance derived from the bonding of molecules. In the case of NBR, the two parts are butadiene and acrylonitrile, which chemists combine using a process known as copolymerization.
These molecules provide specific advantages for the gloves: Acrylonitrile enhances the chemical resistance, while butadiene creates flexibility and tear resistance.
Polyvinyl chloride (PVC) must be produced at a polymer production facility through the polymerization of vinyl chloride monomers. The raw PVC material then receives a plasticizer, making it soft and pliable; otherwise, the PVC would be rigid, as it is when used to form pipe. It is then sent to a glove production facility.
Once the synthetic materials are prepared, they are added to the production process. With a few exceptions—primarily involving washing and chlorination to remove latex proteins—this process is mostly the same as the steps for manufacturing latex gloves.
The last steps of the cycle include testing the gloves, then boxing and shipping them. You can read more about the testing process in our recent post, Acceptable Quality Level Determines If a Glove Is Industrial or Exam Grade.
Poly is a process all its own
Polyethylene is the most affordable glove material and is used primarily in the food service industry.
It is a polymer that is synthesized from ethylene and a thermoplastic that is formed into various shapes as it cools from a liquid state to a solid state.
Two polyethylene sheets are seamed and sealed with heat to create disposable gloves. Because poly gloves are not dipped like latex, nitrile and vinyl gloves, they are not impervious to liquids. Vinyl gloves are a suitable alternative for food services tasks where liquids are present.
Happy Holidays from everyone at AMMEX!